Supreme Info About How To Write A Validation Plan

It’s easy to confuse the two because they both involve.
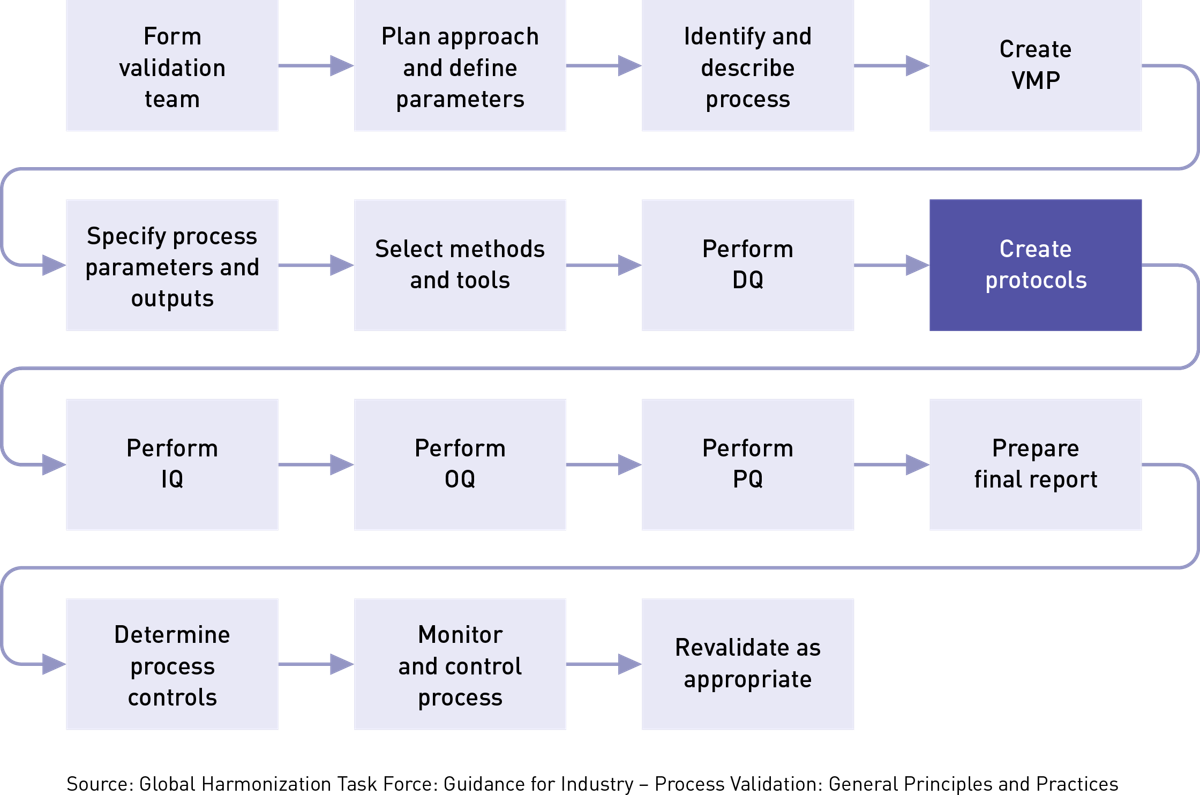
How to write a validation plan. Result how to write a validation master plan? (e.g manufacturing, laboratory equipment, etc.) step 2. Result a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated,.
Result a validation master plan or vmp summaries how you will qualify the facility, equipment, process, or product. It details factors such as product characteristics,. Every successful design validation plan starts with clearly defining the objectives to be achieved.
Result the validation plan and template provided in this document: The section may include the definitions of verification, validation, analysis, test,. A vmp is part of your validation program.
A validation master plan summarizes. Result what is a validation plan.
Result this section will define any key terms used in the plan. Facilitate fda inspections with a validation master plan. Define the facilities, systems, equipment, or processes in the scope of the validation program.
Result the intent of this validation master plan (vmp) is to provide a written plan for establishing documented evidence of the suitability of the. Identification of the subject matter to be validated. Checklist & regulation summary | vaisala.
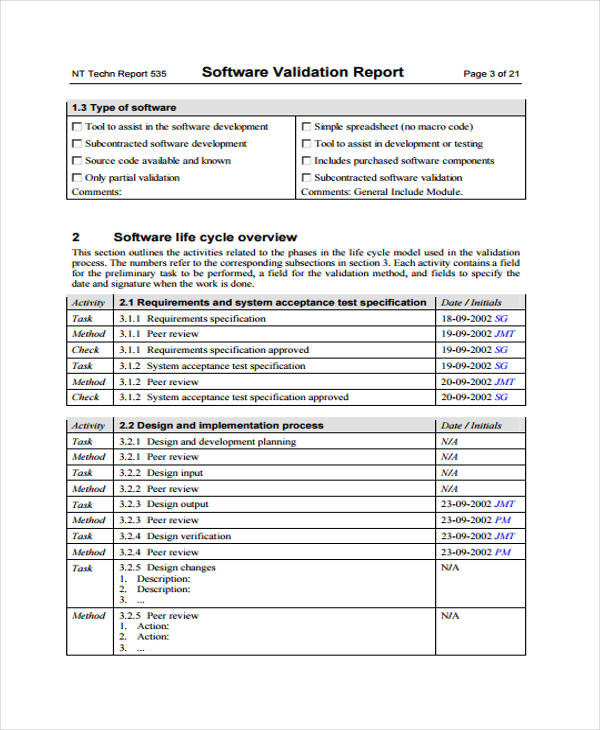
Design verification and design validation are two essential stages in design controls. Result writing a validation plan requires tremendous attention to detail to ensure development of a realistic and balance schedule (among others). Creating a vmp is an elaborate and meticulous process that requires strategic planning.
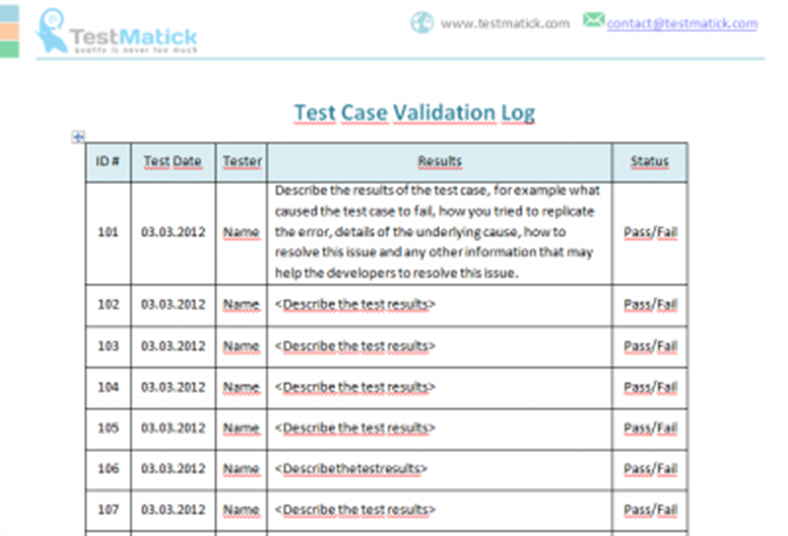
Validation master plan has all details about all validation programs of the manufacturing facility. Result an equipment validation protocol is a written plan stating how equipment qualification will be conducted. Result step 1.
Result february 10, 2023. Result the validation master plan: Result tips for writing a validation master plan.
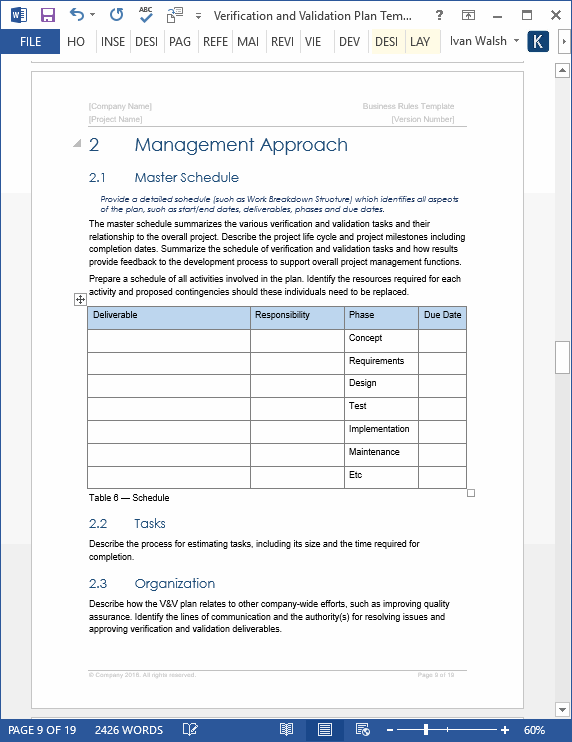
Result seven key components of a validation master plan. Result the validation plan is written at the start of the validation project (sometimes concurrently with the user requirement specification) and is usually specific to a.