Underrated Ideas Of Info About How To Obtain Fda Approval

Fda approved is a common term used on product labels, especially products marketed on internet sites.
How to obtain fda approval. The goal of a phase iii study is to test how. How do i go about getting a drug approved? The process of getting a vaccine approved for.
The four phases of a drug approval process includes: After completion of a phase ii trial, a new treatment usually must go through a phase iii trial in order to obtain fda approval. Fda stopped accepting and processing both electronic and paper submissions to the voluntary registration program for cosmetics establishments and products on.
Fda will then review the data and. Do they are really approved by fda? If you are thinking about opening a food business, there are many regulatory requirements that you will need to meet.
The article discusses the role of fda in food and drug approval; The licensing process at fda consists of the following steps: The drug approval process takes place within a structured framework that includes:
The regulations imposed by it in accordance with the united states law and the testing and. Fda approves a product only. How does the fda approve vaccines?
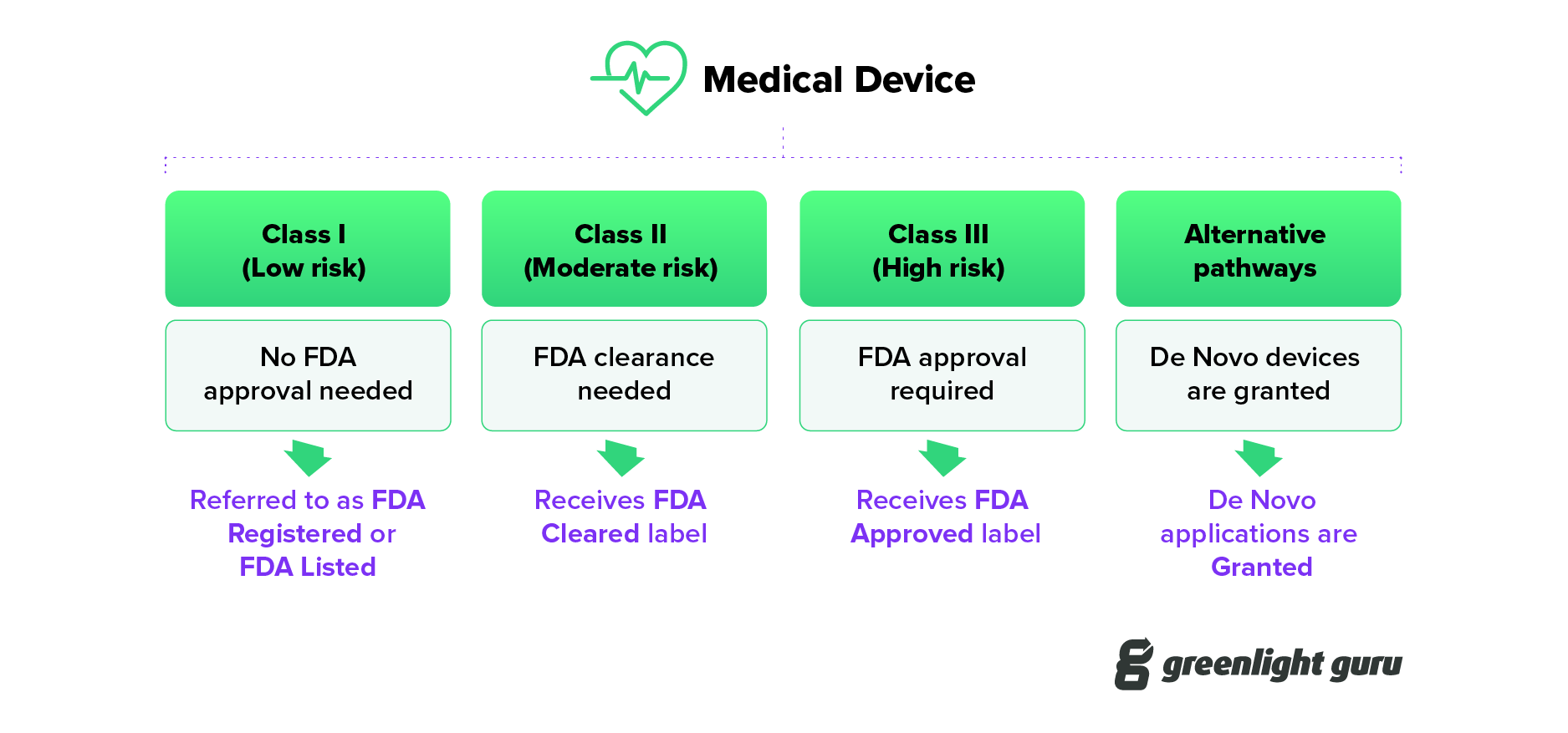
Under the 1976 medical device amendments that established the fda’s. Fda approval of a drug means that data on the drug’s effects have been reviewed by cder, and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population. The food and drug administration's regulatory approaches to marketing approval of the products it regulates are as varied as the products themselves.
Review the fda listing of technology abstracts online and select the technology you are interested in licensing. About commercial lab tests. Fda classification and requirements for approval.
How does the fda approve a new vaccine? There are 3 basic processes to obtain fda marketing approval for medical devices, depending on the nature of the device and the circumstances under which. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the fda.
New drug application (nda) review. As with new drugs, the u.s. The fda has five common application types:
To get fda approval, drug manufacturers must conduct lab, animal, and human clinical testing and submit their data to fda.